The Energy of Activation Is Best Described as
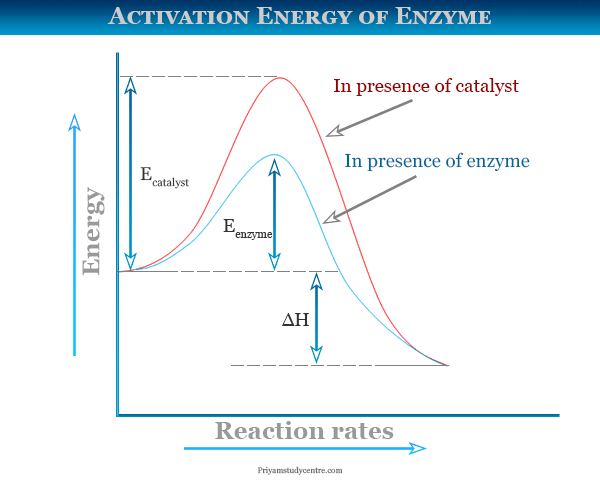
As this graph shows this is the energy which needed by the chemicals reactants for getting the product. It is a threshold value of energy which needs to be crossed for the reaction to occur.

Physical Chemistry Can The Summation Of Activation Energy And Reactant S Initial Internal Energy Change With Temperature Chemistry Stack Exchange
A type of endergonic reaction c.

. D the energy required to separate ions in a crystalline solid. Activation energy is the minimum amount of energy required to initiate a reaction. Activation energy is the difference between the energy of the transition state and the energy of the reactant.
Which best describes the energy of activation. B the minimum kinetic energy that particles must possess for a chemical reaction to occur. The activation energy for popping corn is 167 kJmole 167000 Jmole.
The answer is B as activation energy is the least possible amount of energy minimum which is required to start a reaction or the amount of energy available in a. It can also be described as the minimum amount of energy needed to activate or energize molecules or atoms so that they can undergo a chemical reaction or transformation. The catalysts needed to raise a reactions rate.
Activation Energy The Activation Energy of Chemical Reactions Catalysts and the Rates of Chemical Reactions Determining the Activation Energy of a Reaction The Activation Energy of Chemical Reactions Only a small fraction of the collisions between reactant molecules convert the reactants into the products of the reaction. Which is the best definition of activation energy. Which of the following best describes the activation energy of a reaction.
The meaning of ACTIVATION ENERGY is the minimum amount of energy required to convert a normal stable molecule into a reactive molecule. It is not affected by a catalyst. Even reactions that release energy need a boost of energy in order to begin.
C the energy of motion. The energy needed to start a chemical reaction is called activation energy. It is the height of the potential energy barrier between the potential energy minima of the reactants and products.
A point on the PE diagram where KE PE c. Get 1-on-1 help from an expert tutor now. The transition state is a higher-energy intermediary molecule that lies in between the reactants and products.
The energy of the activated complex b. Activation EnergyEA As the name suggests it is the amount of energy needed for a reaction to occur. The rate at which popcorn pops is described by the Arrhenius equation.
Activation energy is best described as ___ the energy required to initiate a chemical reaction Rank the grades of coal by their relative desirabilities starting with mos desirable at the top. Activation energy is the minimum amount of energy required by chemical reactants to undergo a chemical reaction. The energy required for a reaction to proceed by breaking bonds d.
This is because more energy in the form of heat is available for the reaction to happen. Question thumb_up 100 The rate at which popcorn pops is described by the Arrhenius equation. The energy required for a reaction to proceed by breaking bonds.
A the energy required to remove an electron from a gaseous atom. It is higher if the reaction temperature increases. The higher the activation energy the slower the chemical reaction will be.
The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products. 1 point energy output when product bonds are formed energy input needed to break bonds of reactants energy stored in chemical bonds energy required to. The energy required to form or break the bonds of reactant molecules.
The activation energy of a particular reaction determines the rate at which it will proceed. Activation energy is described as a. A type of exergonic reaction b.
Which definition best describes the term activation energy quizlet. In transition-state theory the activation energy is the difference in energy content between atoms or molecules in an activated or transition-state configuration and the corresponding atoms and molecules in their initial configuration. The activation energy in the Arrhenius equation can best be described as The rate constant for a reaction at 400C is exactly 5 times that at 200C.
Biology questions and answers. The best statement that describes the energy of activation is that it is more quickly overcome when the temperature increases. How many times faster does popcorn pop at 210 degrees C compared with 180 degrees C.
Which statement best describes the energy of activation. Activation energy is the minimum amount of energy needed to start a chemical reaction. The unstable high PE structural arrangement of atoms d.
Activation energy is a concept used in chemistry that was introduced by the scientist from Sweden named Svante Arrhenius in 1889. Calculate the Arrhenius energy of activation for the reaction The rate constant for a. The energy of activation is best described as Multiple Choice ces None of the answer choices are correct the speed at which a reaction proceeds to form products a protein that speeds up a chemical reaction.
Which definition best describes the term activation energy. Transfer Between Systems Quick Check 1Which phrase defines activation energy. Activation energy is also defined as the least possible energy required to initiate a chemical reaction.
Advertisement Answer 47 5 4 22nlin Answer. The minimum amount of additional energy needed by a reacting molecule to get transformed into the product is termed activation energy. It is lower if the reaction temperature decreases.
It is more quickly overcome when the temperature increases. The minimum PE difference between the activated complex and the reactants. Activation energy is defined as the minimum amount of extra energy required by a reacting molecule to get converted into product.
What is activation energy diagram. Activation energy is denoted by E a and typically has units of kilojoules per mole kJmol or kilocalories per mole kcalmol. Recall that activation energy is the energy barrier that needs to be overcome by a reaction.

Kinetics Why Is Activation Energy Drawn In A Potential Energy Diagram In Reactions Chemistry Stack Exchange

No comments for "The Energy of Activation Is Best Described as"
Post a Comment